Stayhealthy Canada is sourcing best in class test kits that provide results rapidly and are approved for use in Canada.
Starting with the BTNX COVID test kit we will be introducing more products over the next few months and years that meet the quality and ease of use criteria Canadians expect
COVID-19 Antigen Rapid Test Device
The Rapid Response™ COVID-19 Antigen Rapid Test Device is an in vitro immunochromatographic assay for the direct and qualitative detection of SARS-CoV-2 viral nucleoprotein antigens from nasal and nasopharyngeal secretions from individuals suspected of COVID-19 within 6 days of symptom onset and from individuals without symptoms or other epidemiological reasons to suspect COVID-19 infection, when tested twice over two (or three) days with at least 24 hours (and no more than 36 hours) between tests. This test is authorized for use at the Point of Care i.e., in patient care setting.

Results are for the identification of SARS-CoV-2 viral nucleoprotein antigen. Antigens are generally detectable in nasopharyngeal and nasal secretions during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories are required to report all positive results to the appropriate public health authorities.
Negative results should be treated as presumptive, and do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient management.
The Rapid Response™ COVID-19 Antigen Rapid Test Device is intended for use by trained laboratory personnel or health care professionals.
Pursuant to section 5 of the Interim Order Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19, made by the Minister of Health on March 18, 2020, the Rapid Response™ COVID-19 Antigen Rapid Test Device is now authorized for sale or importation in Canada. Interim Authorization Number: 321669
PRODUCT INFORMATION
Product Code: COV-19C25
Sample: Nasal / Nasopharyngeal secretions
Format: Cassette
Quantity: 25 Tests/Kit
Time to result: 15 minutes
Storage Condition: 2-30°C/36-86°F
Test Principle: Immunochromatographic Assay
CONTENTS
Individually packed test devices
Extraction Buffer
Extraction tube
Nozzle with filter
Tube stand
Individually packed swabs
Package Insert
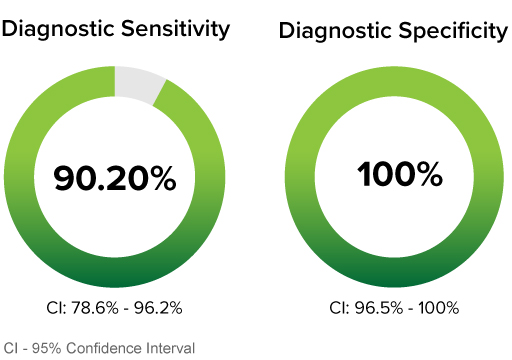
CLINICAL PERFORMANCE WITH NASAL SPECIMENS
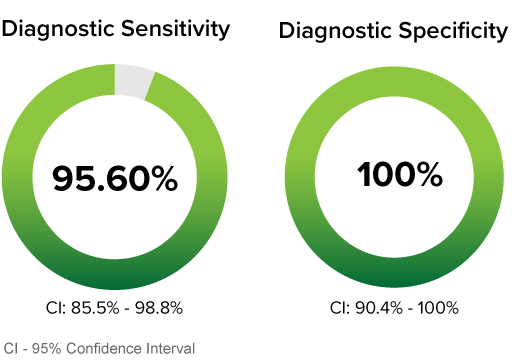
CLINICAL PERFORMANCE WITH NASOPHARYNGEAL SPECIMENS
Copyright 2024 Stayhealthy Canada, Ltd. | All Rights Reserved | Terms of Service